Chinese Scientists Develop Zirconium Nitride Catalysts to Replace Platinum for Oxygen Reduction
The huge consumption of the fossil fuel has led to the environmental pollution and energy crisis. It is foreseeable that, in the near future, the limited carbon-based fossil energy will be replaced by the endless renewable energy, among which the fuel cells and metal-air batteries are the best candidates. However, Pt catalyst used in the fuel cells is so expensivethat account for about 20% of the total cost of the fuel cell. The high cost limits the wide applications of fuel cells. Therefore, the development of low-cost electrocatalytic materials with high activity and stability still remains a great challenge.
Recently, Prof. WANG Jiacheng from Shanghai Institute of Ceramics, Chinese Academy of Sciences, in collaboration with Prof. YANG Minghui from Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Prof. J. Paul Attfield from University of Edinburgh and Prof. Tiju Thomas from Indian Institute of Technology Madras Adyar, discovered that zirconium nitride (ZrN) catalyst based on cheap earth-abundant elements is a highly attractive alternative to Pt for oxygen reduction reaction (ORR) in alkaline environments. ZrN catalyst features low cost, high activity and superior stability. The study was published in the Nature Materials (doi: 10.1038/s41563-019-0535-9) with the title “Zirconium Nitride Catalysts Surpass Platinum for Oxygen Reduction”.
In this study, the scientists produced fine ZrN NPs using a urea–glass route at moderate temperatures, and showed that ZrN can replace and even surpass Pt as a catalyst for oxygen reduction in alkaline environments. In addition, ZrN has a higher stability than the Pt/C catalyst. In a zinc-air battery device, the performance of commercial Pt catalysts deteriorates significantly after a period of time; while the performance of ZrN catalysts deteriorates much more slowly.
In the future, the research team will further cooperate with industry to bring this research outcome into practical use in the clean energy. It is expected to solve the global concerned problems of energy crisis and environmental pollution.
Reference: www.nature.com/articles/s41563-019-0535-9
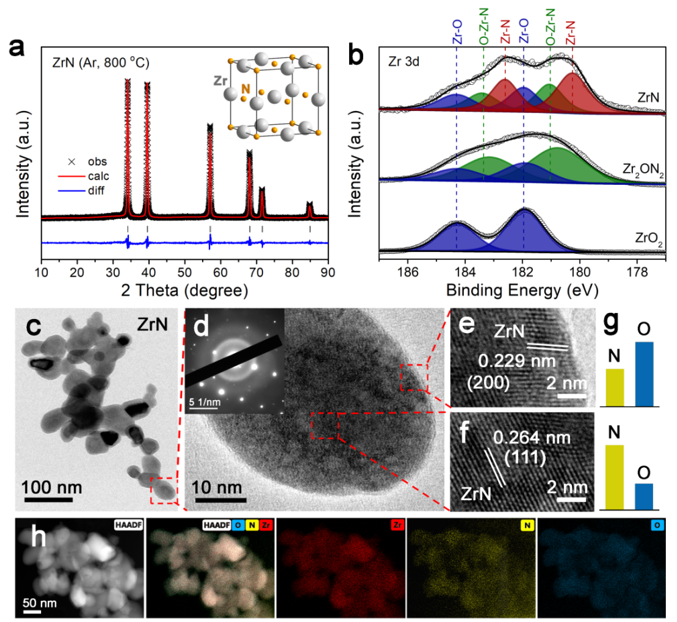
Fig. 1 Characterization of ZrN NPs.
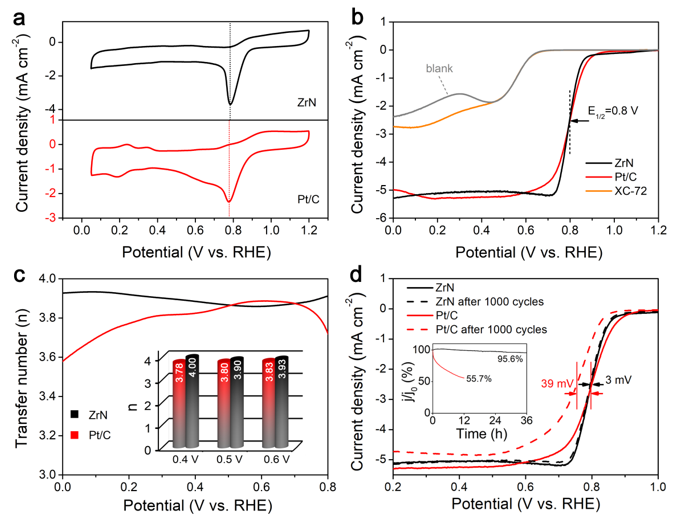
Fig. 2 ORR catalysis properties of nanoparticulate ZrN and Pt/C in an O2-saturated 0.1 M KOH solution.
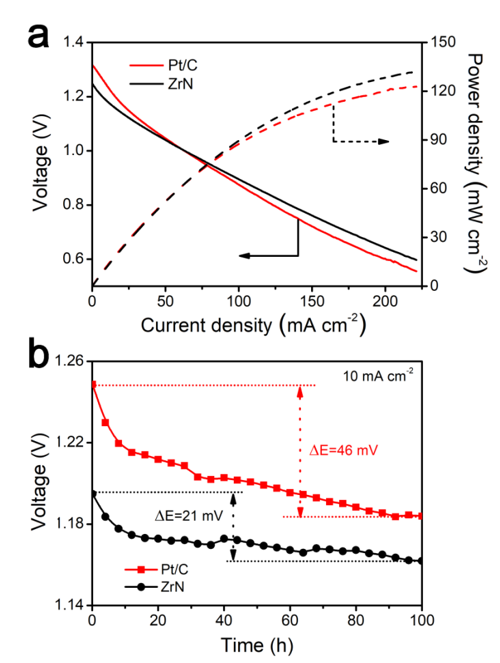
Fig. 3 Zinc–air batteries using nanoparticulate ZrN or Pt/C cathodes.
Contact:
Prof. WANG Jiacheng
Shanghai Institute of Ceramics, Chinese Academy of Science
Email: jiacheng.wang@mail.sic.ac.cn



