- Home
- About Us
- News
-
Research
- State Key Laboratory of High Performance Ceramics
- State Key Laboratory of Functional Crystals and Devices
- The Frontier Department
- Structural Ceramics and Composite Materials
- Information Functional Materials and Devices
- Inorganic Coating Materials
- Artificial Crystal
- Transparent Ceramics
- Energy Materials
- Biomaterials and Tissue Engineering
- Ancient Ceramics and Industrial Ceramics
- Pilot Test for New Materials
- People
- International Cooperation
- Education
- Journals
- Links
A New Strategy: Photocatalysis to Realize the Methane-to-Ethanol Conversion
As the main component of natural gas and shale gas, methane has the advantage in its abundant reserve and low cost. Instead of petroleum, methane is a competitive candidate for producing the liquid fuels and value-added chemicals, and has attracted great interests from the academic and industrial communities. The oxygenated derivatives of methane, especially the alcohol derivatives, are deemed as the pillar of C1 chemistry. Methane is the most stable organic molecule with high C-H bond strength (~440 kJ mol-1). However, the intermediates after C-H activation are highly reactive and hard to escape from deep mineralization.
The selective methane activation and its oriented conversion become the global challenges in the academic fields of the catalysis as well as the chemistry. By now, the industry still uses the indirect method of methane valorization, which depends on preliminary high-temperature and high-pressure oxidation to produce syngas. Then the liquid fuels and commodity chemicals are produced through the Fischer-Tropsch process. The entire process is low efficient with no more than 50% of the carbon conversion, while it causes environmental pollution for enormous CO2 emissions.
Therefore, many researchers are dedicated to exploring the direct methane conversion method to reduce the energy consumption.
Photocatalysis promotes the reactions under the mild conditions by virtue of photoexcitation instead of thermal activation, which becomes a promising green alternative for direct methane conversion.
Recently, Prof. Wenzhong Wang and his group from Shanghai Institute of Ceramics have made new progress in the research of photocatalytic methane conversion. They designed Cu modified polymeric carbon nitride, which achieved photocatalytic methane-to-ethanol conversion. They also made in-depth explorations in the photocatalytic mechanism. The result was published in Nature Communications (DOI: 10.1038/s41467-019-08454-0) entitled ‘Direct functionalization of methane into ethanol over copper modified polymeric carbon nitride via photocatalysis’. A Chinese invention patent was also granted (201811339733.2).
Given that both the excessive hydroxyl radical from direct H2O oxidation and the neglect of methane activation on the material will cause deep mineralization, the research group introduced Cu species into polymeric carbon nitride. They realized photocatalytic anaerobic methane conversion with an ethanol productivity of 106 μmol g-1 h-1. Cu modified polymeric carbon nitride could manage generation and in situ decomposition of H2O2 to produce hydroxyl radical, of which Cu species were also active sites for methane adsorption and activation. These features avoided excess hydroxyl radical for overoxidation and facilitated methane conversion. Moreover, a hypothetic mechanism through a methane-methanol-ethanol pathway was proposed, emphasizing the synergy of Cu species and the adjacent C atom in the polymeric carbon nitride for obtaining C2 product. This work put forward a strategy to construct a mild condition for methane activation, broadening the horizon of methane conversion.
The work was supported by the National Natural Science Foundation.
Article link: https://www.nature.com/articles/s41467-019-08454-0
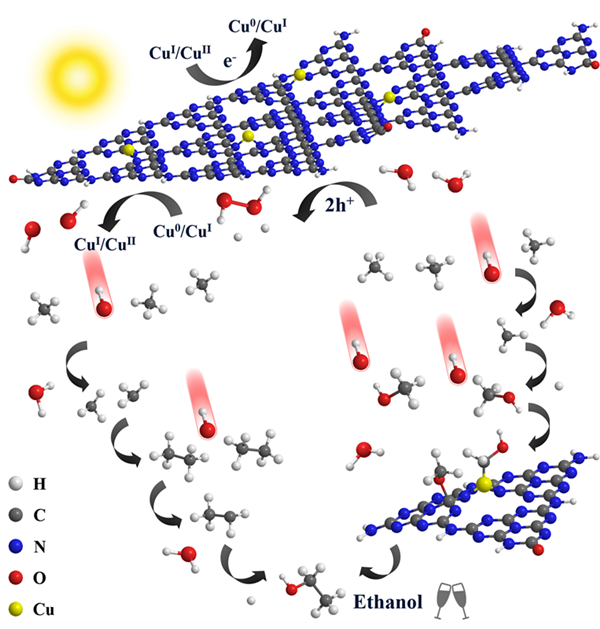
Photocatalytic methane conversion over Cu modified polymeric carbon nitride
Contact:
Prof. Wenzhong Wang
Shanghai Institute of Ceramics
wzwang@mail.sic.ac.cn



