Innovative Catalyst Advances Acidic Water Splitting Technology
Quinary High-Entropy Ruthenium Iridium-Based Oxide Holds Promise for Large-Scale Application in PEMWE
Researchers from the Shanghai Institute of Ceramics of the Chinese Academy of Sciences(SICCAS), in collaboration with Xiamen University and others, have made a significant breakthrough in electrocatalytic water splitting, a key technology for converting intermittent solar and wind energy into clean hydrogen fuel.
In the pursuit of a hydrogen society, electrocatalytic water splitting has emerged as a potential solution. However, the acidic operating environment of PEM has presented challenges for the long-term use of ruthenium oxide (RuO2). Now, a team led by Prof. WANG Xianying at SICCAS has discovered a quinary high-entropy five-membered ruthenium iridium-based oxide (M-RuIrFeCoNiO2), offering promising applications in PEMWE.
The results were published in Science Advances. The team developed a unique synthesis strategy for M-RuIrFeCoNiO2, creating abundant grain boundaries (GB). This innovation significantly enhances the catalytic activity and stability of RuO2 in acidic oxygen evolution reactions (OER), overcoming previous limitations.
The deliberate integration of foreign metal elements and grain boundaries within the oxide catalyst played a pivotal role in improving OER activity and stability. This groundbreaking approach effectively resolves thermodynamic solubility issues associated with different metal elements.
Practical application tests showed remarkable results, as a PEMWE employing the M-RuIrFeCoNiO2 catalyst sustained a large current density of 1 A cm-2 for over 500 hours. This achievement marks a significant advancement in PEMWE technology and offers promise for clean hydrogen fuel production at scale.
The research not only showcases a novel synthesis strategy for high-entropy oxides but also provides valuable insights into their activity and stability in the context of PEMWE, contributing to the advancement of clean energy solutions.
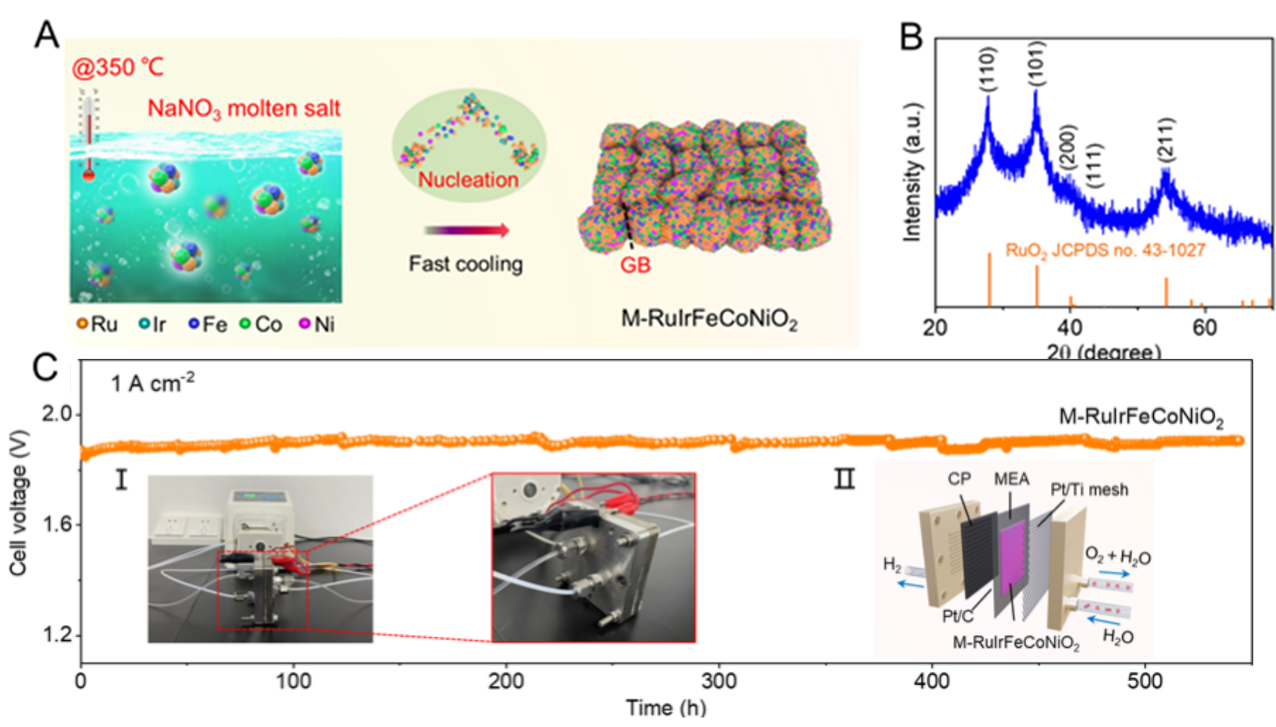
a. Schematic illustration of the fast, non-equilibrium synthetic process for synthesizing M-RuIrFeCoNiO2, showing the generation of GB; b. X-ray diffraction pattern of M-RuIrFeCoNiO2; c. Chronopotentiometric response of M-RuIrFeCoNiO2 at 1 A cm-2 in the PEM electrolyzer. (Image by HU chun)
Contact
Wang Xianying
Shanghai Institute of Ceramics
E-mail: wangxianying@mail.sic.ac.cn
Reference
Misoriented high-entropy iridium ruthenium oxide for acidic water splitting



