Scientists Develop In Situ Ion-Exchange Preparation Methods and Reveal Topological Transformation of Trimetal–Organic Frameworks for Efficient Electrocatalytic Water Oxidation
Developing anion exchange membrane water electrolysis (AEMWE) is a promising route for advancing industrial hydrogen production. However, its slow and complex kinetics of anodic oxygen evolution reaction (OER) largely determines the energy consumption for overall water electrolysis. Currently, the Ir and Ru-based noble metals composites (e.g., RuO2 and IrO2) show high catalytic activities for OER, but the high cost, scarcity, and poor stability restrain their commercially large-scale applications. Consequently, it is highly desiderated yet challenging to develop highly efficient and resource-rich alternative electrocatalyst for OER in AEMWE.
Benefiting from highly dispersed active sites, defect-rich edges, abundant open channels, and nanometer thickness, metal-organic frameworks (MOFs), organized by the periodic coordination of metal nodes/clusters with organic ligands, have demonstrated promising electrochemical catalytic activities. However, these MOFs systems reported so far still bear intrinsically low activity and poor stability, becoming a significant bottleneck limiting their direct utilization in the OER electrocatalysis.
Except for the morphology engineering, introducing heteroatom units in a primitive MOF nanostructure would an effective strategy to address these issues. It can regulate intrinsic charge ordering to optimize the electronic structure of the host metal, resulting in the enhancement of catalytic performance.
Recently, the research team led by Prof. WANG Xianying from Shanghai Institute of Ceramics, Chinese Academy of Sciences has made progress in the application of MOF for water oxidation. This work reports the synthesis of ultrathin NiCoFe-NDA (NDA=2,6-naphthalenedicarboxylic acid) nanosheets through a simple redox-precipitation approach for efficient OER electrocatalysis in AEMWE. Compared with monometal (Ni/Co/Fe)-NDA/NF, the NiCoFe-NDA/NF electrode exhibits a much higher OER activity and stability, which even exceeds the benchmarked IrO2 catalyst. As the anodic electrode, this NiCoFe-NDA affords a cell voltage of 1.8 V to stably drive the current density of 325 mA cm-2 in a home-made AEMWE for over 100 h continuous operation.
The related result is published on Energy & Environmental Science (2021, 14, 6546–6553) entitled " In situ ion-exchange preparation and topological transformation of trimetal–organic frameworks for efficient electrocatalytic water oxidation".
This work survey reveals the NiCoFe-NDA/NF suffers a topotactical transformation and surface reconstruction. The inherited unsaturated coordination environment and trimetal coupling effects are proved to be positive for enhancing the OER performance. This work not only demonstrates an ideal platform for the correction of multi-metal coordination with water oxidation performance in organic-inorganic hybrid materials but also provides a highly promising electrocatalyst for water oxidation and other related energy technologies.
This project is funded by the Start-up Foundation of Shanghai Institute of Ceramics, Chinese Academy of Sciences, the National Natural Science Foundation of China and the National Key Basic Research Program of China.

Schematic illustration of the formation and transform process of NiCoFe-NDA/NF.
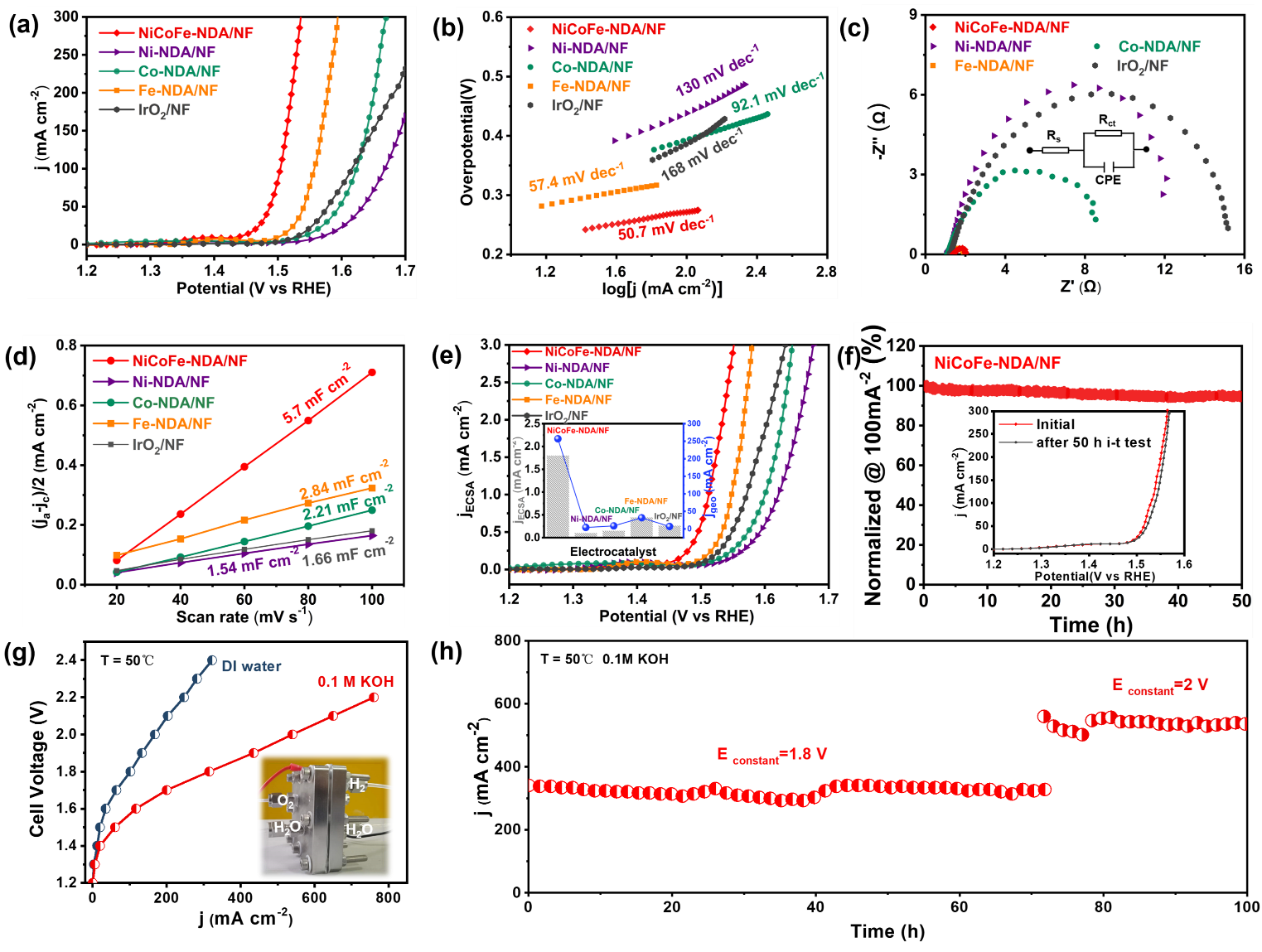
Water oxidation performance of NiCoFe-NDA and its performance in anion-exchange membrane water electrolysis
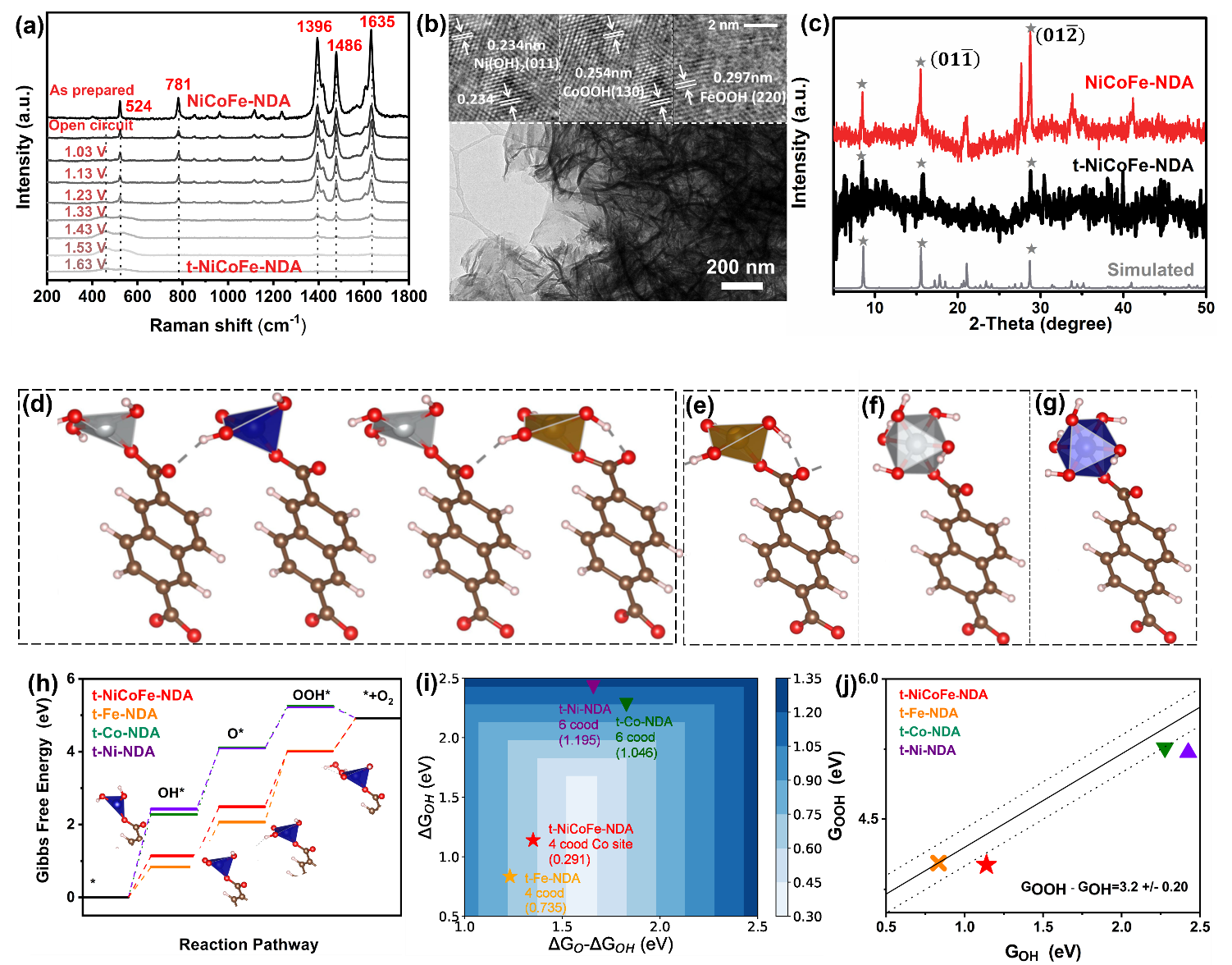
In situ spectroscopy combined with theoretical calculations to illustrate the water oxidation
conformation of NiCoFe-NDA and the study of the water oxidation mechanism
Reference
https://doi.org/10.1039/D1EE02606B
Contact:
Prof. WANG Xianying
Shanghai Institute of Ceramics
Email: wangxianying@mail.sic.ac.cn



