A New Strategy for Resisting the Deactivation of the Photocatalyst during VOCs Photodegradation
Volatile organic compounds (VOCs) such as aldehydes, ketones, and aromatics are the products from some natural processes and industrial operations. The exposure to VOCs will cause serious health issues, i.e., nausea, respiratory problems, dizziness, liver damage, and cancer. In addition, VOCs are accountable for the formation of urban smog and the depletion of ozone layer in the troposphere. Therefore, it is indispensable to develop techniques for safe removal of VOCs from the atmosphere.
Among the treatment technologies, photocatalytic oxidation (PCO) has been widely considered for the prosperous pollution control technique. Also, TiO2 photocatalyst is one of the most promising photocatalyst for industrial application due to its non-toxicity, high oxidation potential and good photochemical stability. However, one crucial problem encountered in the real application is the catalysts deactivation, which is mainly resulted from the partial for full coverage of active sites by intermediates in VOCs degradation.
The research group led by Prof. SUN Jing at Shanghai Institute of Ceramics of the Chinese Academy of Sciences synthesized photocatalyst rGO/Er3+-TiO2 with controllable microstructure and enhanced photoelectric properties, based on the special 4f electronic structure of rare earth elements and the characteristics of rGO with good conductivity, large specific surface area and abundant π-bond (similar to benzene ring). The photocatalyst rGO/Er3+-TiO2 shows good photocatalytic performance in VOCs degradation and inhibiting catalyst deactivation. The result was published in Applied Catalysis B: Environmental (Appl. Catal. B, 284 (2021) 119813) and applied for an invention patent.
The photocatalyst rGO/Er3+-TiO2 was prepared by “two-step” method and showed high photocatalytic performance and excellent durability for o-xylene (< 100 ppm). The degradation efficiency towards 25 ppm o-xylene reached 100%, and there was no obvious deactivation after 40 h of photocatalytic reaction. However, the photocatalytic activity of TiO2 and Er3+-TiO2 samples was significantly inactivated after 60 min. The key reason for the deactivation of the catalyst was that the intermediates accumulated on the surface of the catalyst and occupied the reactive sites. This study revealed that the introduction of rGO provid additional adsorption sites both for the initial adsorption of VOCs molecules and also for accommodating the generated intermediates. Thus, the original reactive sites were well kept from being covered by pollutants and can produce free radicals continuously, thereby leading to long-term activity of the photocatalyst. This work provides a promising strategy for preventing catalyst deactivation during gas-phase o-xylene degradation and a new insight into the deactivation and activation mechanism of the photocatalyst.
The research was supported by the National Key Research and Development Program of China (2016YFA020300) and the National Natural Science Foundation of China (41907303).
Reference:https://doi.org/10.1016/j.apcatb.2020.119813

(a) The degradation and (b) CO2generationcurves for o-xylene with 0.1g sample illuminated by a 400 W xenon lamp, and (c) histogram of photocatalytic efficiency and mineralization rate.
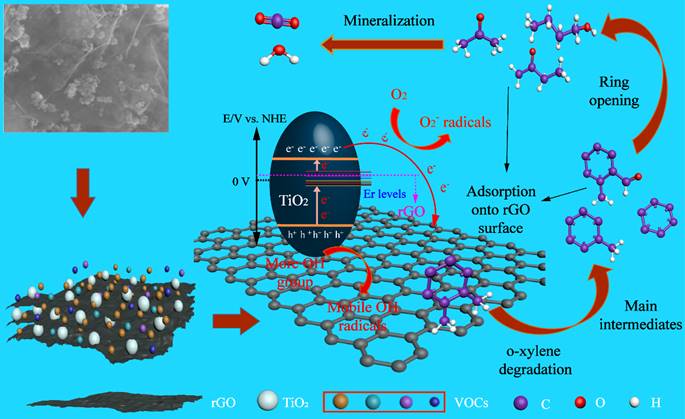
Schematic diagram of enhancing photocatalytic activity and the proposed reaction routes for VOC photodegradation. rGO provides additional adsorption sites for accommodating intermediates to resist the deactivation of the photocatalyst.
Contact:
Prof. SUN Jing
Shanghai Institute of Ceramics
Email: jingsun@mail.sic.ac.cn



