Enhanced Performance of In-Plane Transition Metal Dichalcogenides Monolayers by Configuring Local Atomic Structures
The research group from Shanghai Institute of Ceramics, led by Prof. LIU Jianjun, cooperated with Nanyang Technological University of Singapore, theoretically designed the triangular-shape cobalt atom cluster with a central sulfur vacancy embedded the basal plane of 2H-MoS2 (3CoMo-Vs) as hydrogen evolution reaction (HER) catalyst. The research group was the first to propose the idea of local configuration electronegativity to study the intrinsic activity of 2H-MoS2. It was verified that the activity of in-plane sulfur of 2H-MoS2 can be regulated by electron transfer capacity of local configurations (3CoMo-Vs) in experiment and theory. The research was published in Nature Communications (https://www.nature.com/articles/s41467-020-16111-0).
MoS2 is a promising candidate to replace Pt for electro-catalytic hydrogen evolution reaction due to its environmental friendly and low cost characteristics. However, the reported activity of in-plane sulfur of 2H-MoS2 is still far from that of Pt-based catalysts. In particular, changing the local configurations by introducing atomic defects (doping or vacancy) is preferable as the defected MoS2 exhibits better stability and activity compared to the other strategies. In fact, few types of atomic local configurations have been realized in the basal plane of MoS2, thus the tuning ability of local configurations is quite limited so far. In order to improve the intrinsic activity of in-plane sulfur atoms, it is essential to theoretically design local configurations and experimentally explore new methodologies to realize stable and highly efficient catalyst.
The research group found that the hydrogen bind strength and local triangular-shape cobalt atom cluster with a central sulfur vacancy renders the distinct electro-catalytic performance of MoS2 with much reduced overpotential (75 mV at 10 mA cm-2) and Tafel slope (57 mV dec-1). In addition, they systematically studied the four kinds of doping defects and boundary structures of MoS2 (transition metal atoms replace Mo, S-vacancies, Mo-edges and S edges). The regional electronegativity (Ψ) is proposed as a catalytic activity descriptor, which could screen the highly efficient catalyst. Related results were published in Chemistry of Materials. https://pubs.acs.org/doi/10.1021/acs.chemmater.9b04377
The referee praised as “I want to applause the huge effort…this is a very solid piece of work that deserve publication in its present form.” (cited from Peer Review File, https://www.nature.com/articles/s41467-020-16111-0)
This work was supported by projects such as the National Natural Science Foundation of China.
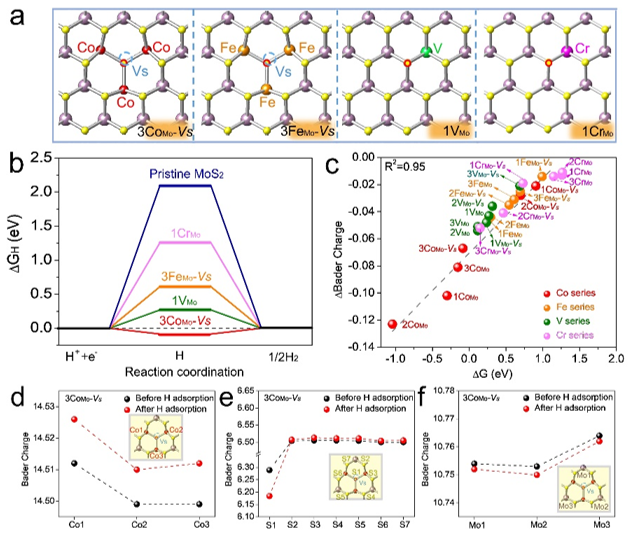
Figure 1. (A) The most stable structures of MoS2 with 3CoMo-Vs, 3FeMo-Vs, 1VMo, and 1CrMo configurations and the S bonding with H is marked as red circles. (B) The free energy diagram of corresponding configurations and pristine MoS2. (C) The correlation between change of Bader charge of local configuration around sulfur atoms and hydrogen adsorption free energy (ΔGH). The dashed line is linearly fitted with R2=0.95. The Bader charge changes of (D) Co atoms, (E) S1 and next-neighbor S atoms, and (F) the next-neighbor Mo atoms when 3CoMo-Vs is introduced in the MoS2.
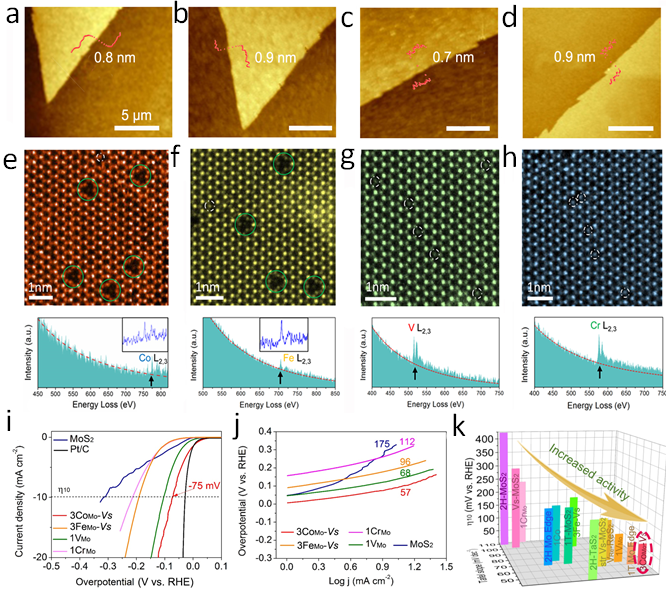
Figure 2. AFM images of (A) 3CoMo-Vs, (B) 3FeMo-Vs, (C) 1VMo, and (D) 1CrMo samples, illustrating the monolayer nature of the as-synthesized MoS2. (E–H) Atomic resolution STEM images of the TM-containing MoS2 samples with corresponding electron energy loss spectrum on single TM. (I) Polarization curves of pristine MoS2, MoS2 with 3CoMo-Vs, 3FeMo-Vs, 1VMo, 1CrMo configurations and Pt/C. The currents are normalized to the projected geometric area of the electrode. (J) The corresponding Tafel plots of the polarization curves in I. (L) Comparison of η10-Tafel slope for HER catalysts in 0.5 M H2SO4. MoS2 with 3CoMo-Vs configuration exhibits top performance. The data are taken from refs.
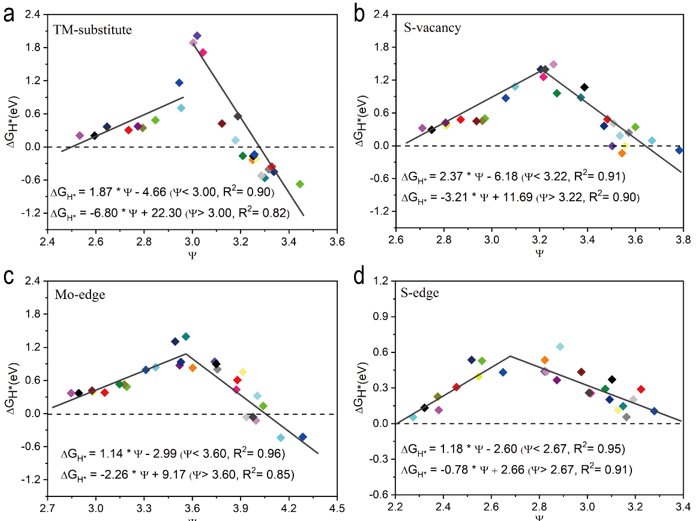
Figure 3. Curve fitting of HER with Ψ supported on MoS2 with different defect structures. (A) Perfect MoS2, (B) MoS2 with S vacancy, (C) MoS2 with Mo-edge structure, and (D) MoS2 with an S-edge structure.
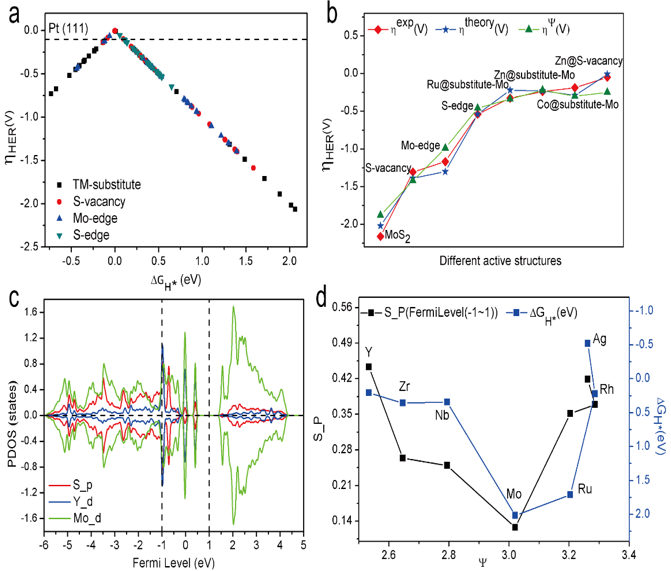
Figure 4. Validation of catalytic descriptors. (A) HER theoretical overpotential versus adsorption free energies ΔGH* for all TMs doped in MoS2 with different defect structures. (B) Theoretical and corresponding experimental and calculated by descriptor overpotentials for HER versus the descriptor Ψ. (C) PDOS of Y@substitute-Mo. (D) Active electron number based on the projected density of states (PDOS) for the 4d transition metal doping in the TM-substitute structure and adsorption free energies ΔGH* versus the descriptor Ψ.
Article link: https://www.nature.com/articles/s41467-020-16111-0.
Contact:
Prof. LIU Jianjun
jliu@mail.sic.ac.cn



