Mg-based batteries get progress in Dual-salt hybrid system with conversion cathodes and transition-metal free system activated by anionic insertion
Mg batteries, as the most typical multivalent batteries, are attracting increasing attention because of the resource abundance, high volumetric energy density, and smooth plating/stripping of Mg anodes. However, current limitations for the progress of Mg batteries come from the lack of high voltage electrolytes and fast Mg-insertable structure prototypes. The commercially available carbonic ester electrolytes would passivate Mg anode, and cannot be used in Mg batteries. Although Mg2+ (0.86 ?) has a similar ionic radius as that of Li+ (0.9 ?), its high charge density and polarity usually lead to a low solid-diffusion rate of Mg2+ in most of the crystal structures.
Recently, the research group led by Prof. Chilin Li from Shanghai Institute of Ceramics, Chinese Academy of Sciences has developed Mg-based battery systems and their reaction mechanisms.
The researchers proposed a dual-salt Mg-based battery characterized by high capacity conversion reaction at cathode (Figure 1). This system combines the advantages of kinetically favorable Li-driven conversion of FeSx (with 2-4 electron transfer) and smooth (dendrite-free) Mg plating/stripping by using a dual-salt Mg2+/Li+ electrolyte. It simultaneously bypasses sluggish Mg-ion diffusion in host lattice at the cathode, lithium dendrite propagation at the anode as well as alleviates polysulfide dissolution in electrolyte. The combination of FeS2 and Mg render this hybrid battery a much higher energy density close to 400 Wh/kg. In situ formation of solid electrolyte interfaces on both sulfide and Mg electrode is likely responsible for long-life cycling (e.g. reversible capacity of more than 200 mAh/g preserved after 200 cycles) and suppression of S-species passivation at Mg anodes. See the publication Adv. Funct. Mater. 2015, DOI: 10.1002/adfm.201503639.
The researchers also proposed to utilize a fast redox process at surface decoration groups of ultrathin nanostructures to replace sluggish lattice migration for improving the kinetics of Mg batteries (Figure 2). Taking fluorinated graphene nanosheets as model material, a reversible capacity higher than 100 mAh/g is achieved in a pseudocapacitance behavior. Different from traditional storage mechanisms, this proof-of-concept Mg-based battery system is activated by a prior anionic process followed by reversible cationic storage. The dilution of charge density by forming large-sized monovalent complex cations and the easy access to surface redox sites are responsible for the negligible voltage polarization without an evident MgF2 nucleation phenomenon. See the publication Adv. Funct. Mater. 2015, 25, 6519-6526.
These works are supported by National Natural Science Foundation of China and China Postdoctoral Science Foundation.
Article link:
http://onlinelibrary.wiley.com/doi/10.1002/adfm.201503639/abstract
http://onlinelibrary.wiley.com/doi/10.1002/adfm.201503010/abstract
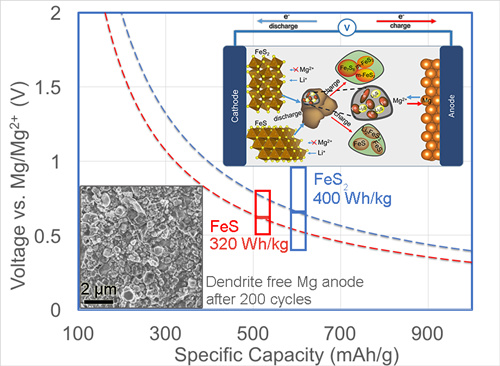
Figure 1: Dual-Salt Mg-Based Batteries with Conversion Cathodes
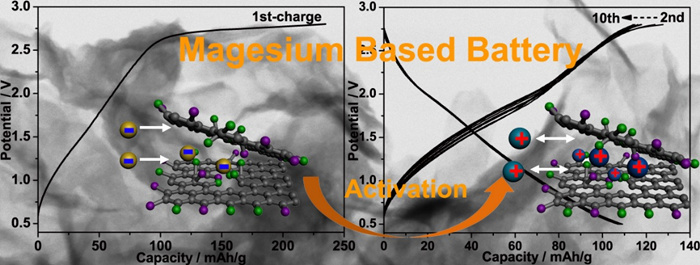
Figure 2: Transition-Metal-Free Magnesium-Based Batteries Activated by Anionic Insertion into Fluorinated Graphene Nanosheets



