Scientists Develop Novel design strategies toelectrolyte and electrode materials based on molecular orbital energy level modulation
A research team led by Prof. Xiaowei Chi and Prof. Yu Liu from the Shanghai Institute of Ceramics of the Chinese Academy of Sciences has introduced pioneering design strategies for electrolyte and electrode materials based on molecular orbital energy level modulation. These approaches have significantly enhanced the energy density and cycle life of existing aqueous zinc-based batteries and iron-based flow batteries by several fold.
Their findings, published in Advanced Materials and the Journal of the American Chemical Society, under the titles "A 1000 Wh kg−1 Cathode Facilitated by In Situ Mineralized Electrolyte with Electron Potential Well for High-Energy Aqueous Zinc Batteries" and "Molecular Tailoring of Iron Chelates for Long-Cycling and High-Efficiency All-Iron Redox Flow Batteries". The authors of the publications are Ph.D. candidates Lingbo Yao and Mingda Luo, respectively, under the supervision of Professors Yu Liu and Xiaowei Chi.
(https://doi.org/10.1002/adma.202505342,https://doi.org/10.1021/jacs.5c11383)
Aqueous metal batteries, particularly those based on zinc and iron, have emerged as promising next-generation energy storage solutions due to their unique advantages in safety and cost-effectiveness. They are considered highly potential for applications in large-scale grid storage and backup power systems. However, challenges such as metal dendrite formation and hydrogen evolution at the anode, as well as material dissolution and shuttling effects at the cathode, significantly impact their energy density and cycle life, hindering their commercial applications.
The team’s novel approach focuses on In-situ Mineralized Electrolyte and Electron Potential Well-mediated High-energy and Long-life Aqueous Zinc Metal Batteries:Inspired by biomineralization phenomena in nature. They designed an in-situ mineralized electrolyte using Prussian blue analogues as mineralizing agents, which function as electron potential wells to synergistically modulate the energy level structures of both the Zn anode and halogen cathode, thereby promoting uniform, rapid, and reversible charge transfer at the electrode/electrolyte interface. On the cathode side, this electron potential well enables the iodine cathode to achieve a specific capacity of 286.4 mAh g−1 at a current density of 1 A g−1 and a cycle life of 2,000 cycles, while on the anode side, it facilitates the in-situ reduction of zinc dendrites into active Zn2+, ensuring stable cycling of the zinc anode for 1,500 hours under a practical areal capacity of 5 mAh cm−2. Moreover, the electron potential well mediates synergistic iodine‑bromine chemistry, effectively promoting a highly reversible multi‑electron transfer reaction that delivers a record‑high cathode energy density of 1,143.9 Wh kg−1 compared with the reported literature, with the full cell maintaining an energy density of 503 Wh kg−1 and a high energy efficiency of 86.73% over 6,000 cycles. This study not only pioneers the concept of an "electron potential well" electrolyte but also realizes it through an ingenious in-situ mineralization strategy, simultaneously addressing the key challenges at both electrodes of aqueous zinc metal batteries and achieving a synergistic enhancement in energy density and cycle life, thereby providing a new direction for developing next‑generation high‑performance, low‑cost, and safe aqueous batteries and accelerating their commercialization process for large‑scale energy storage applications.
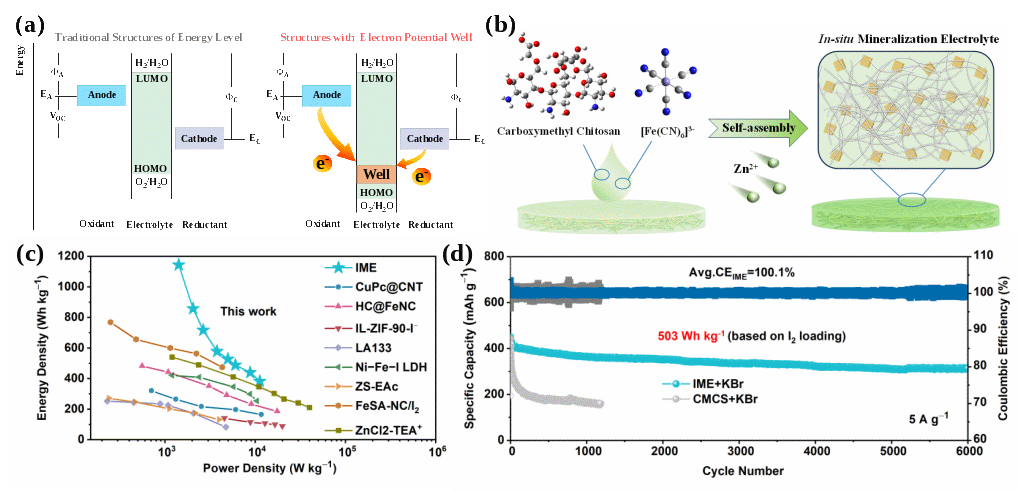
Schematic illustration of the electron potential well-mediated mechanism in the in-situ mineralized electrolyte (a-b) and electrochemical performance of high-energy aqueous zinc-based batteries (c-d).
Molecular Tailoring of lron Chelate for Long-cycling and High-efficiency All-iron Redox Flow Batteries: At the anode side, to address the instability of traditional iron chelates such as Fe-TEA and Fe-THEED, which are prone to chelates decomposition and ligands shuttling, the team designed a novel ligand, 2-hydroxy-1,3-propanediamine tetra-sulfonate sodium salt (HPDTS), through precise molecular tailoring. This innovation enables simultaneous regulation of the ligand structure and chelate configuration, resulting in a highly stable and reversible symmetric hexa-coordinate iron chelate anode. This design not only retains the low redox potential of traditional penta-coordinate iron chelates but also features a symmetric hexa-coordinate structure with shorter Fe-O bond lengths and a higher LUMO energy level. This fundamentally enhances the stability of the chelates, addressing the critical issues of iron dendrite formation and hydrogen evolution caused by the decomposition of anode chelates. Using the Fe-HPDTS chelate as the anode material and commercial iron cyanide as the cathode, a stable 1.3 V aqueous iron-based flow full cell was constructed. Additionally, owing to the larger molecular volume and higher negative charge of this anode material, it exhibits excellent ligand selectivity. When paired with a cost-effective and high-conductivity non-fluorinated proton exchange membrane (PBI), the iron-based flow battery demonstrates outstanding performance: high energy efficiency of 71.79% at 200 mA cm−2, high power density of 446.9 mW cm−2, and long cycle life exceeding 15,000 cycles with a capacity decay rate of only 0.00074% per cycle and high average coulombic efficiency of ~100%. This research not only develops a high-performance iron chelate but also proposes a new paradigm of molecular engineering based on the synergistic design of "ligand structure-chelation configuration", laying a solid foundation for the practical application of iron-based flow batteries. This technology holds great promise for grid-level energy storage, renewable energy integration, and other fields, driving the advancement of long-duration energy storage technologies.

Molecular structure and design principle of anode materials in the iron-based flow battery (a-b) and electrochemical performance of flow battery (c-f).
Contact: Prof. Xiaowei Chi, Prof Yu Liu
Shanghai Institute of Ceramics Chinese Academy of Sciences



