A New Solid Electrolyte Promises High Rate and Interfacial Stability for All Solid-State Battery
Recently, the research group of Prof. LIU Jianjun from Shanghai Institute of Ceramics, cooperating with the research group of Prof. TANG Weiping from Shanghai Institute of Space Power-Sources, designed a high-performance solid electrolyte of Li3Zr2Si2PO12 (LZSP) with space group of C12/c1 (No. 15) from the Na3Zr2Si2PO12 (NZSP) precursor through a skeleton-retained cationic exchange approach. This study proposed a strategy of relocating diffusion ions to under-coordination sites to enhance ionic conductivity of solid electrolyte in experiment and theory, and opened an innovative approach to produce new materials.
The study was published in Science Advances.
Solid electrolytes are the important materials for improving safety, energy density and reversibility of electrochemical energy storage batteries. Although some sulfide solid electrolytes, such as Li10GeP2S12, perform high ionic conductivity of ~ 10-2 S·cm-1, the low stability against Li metal leads to a fast decrease of ionic conductivity to 10-4 S·cm-1.
Other solid electrolytes, such as oxide solid electrolyte with overall stability (electrochemical, chemical and environmental), show low ionic conductivity of <10-3 S·cm-1, hampering the further application. The low ionic conductivity in oxide solid electrolytes is intrinsically ascribed to the narrow bottleneck of Li-ion migration from octahedral occupancy to tetrahedral occupancy.
Therefore, achieving a high ionic conductivity for oxide solid electrolyte has been a long-term and difficult task due to the limit of the bottleneck size.
The research group proposed a strategy of skeleton-retained cationic exchange (Na+ →Li+) to synthesize LZSP material in experiment and revealed a coordination self-adaptation mechanism for Li ions to occupy under-coordination sites and inherit the wide bottleneck from NZSP precursor, which plays the key role in enhancing ionic conductivity, in theory.
This cationic exchange of Na+ →Li+ was experimentally and theoretically proved to make Li-ion occupied in tetrahedral sites in order to maintain structural stability and the migration bottleneck sizes of Li ions are significantly increased due to inherited structure from Na-ion solid electrolyte.
As a result, the synthesized LZSP was demonstrated to not only have the highest Li-ion ionic conductivity of 3.59 × 10-3 S·cm-1 at room temperature in oxide solid electrolytes, but also present considerably stability for Li metal and air. A NCM523/LZSP/Li cell performs a discharge specific capacity of 162.0 mAh·g-1 in the first cycle at 0.1 C and a minor capacity fading of 9.9% after 100 cycles.
The proposed method of skeleton-retained cationic exchange in this study is different from commonly adopted molten salt ion-exchange method, by which phase transformation may take place and result in low ionic conductivity, and could be commonly used to produce more solid electrolyte, even extensive inorganic materials, by controlling suitable reaction conditions.
Reference: https://doi.org/10.1126/sciadv.abj7698
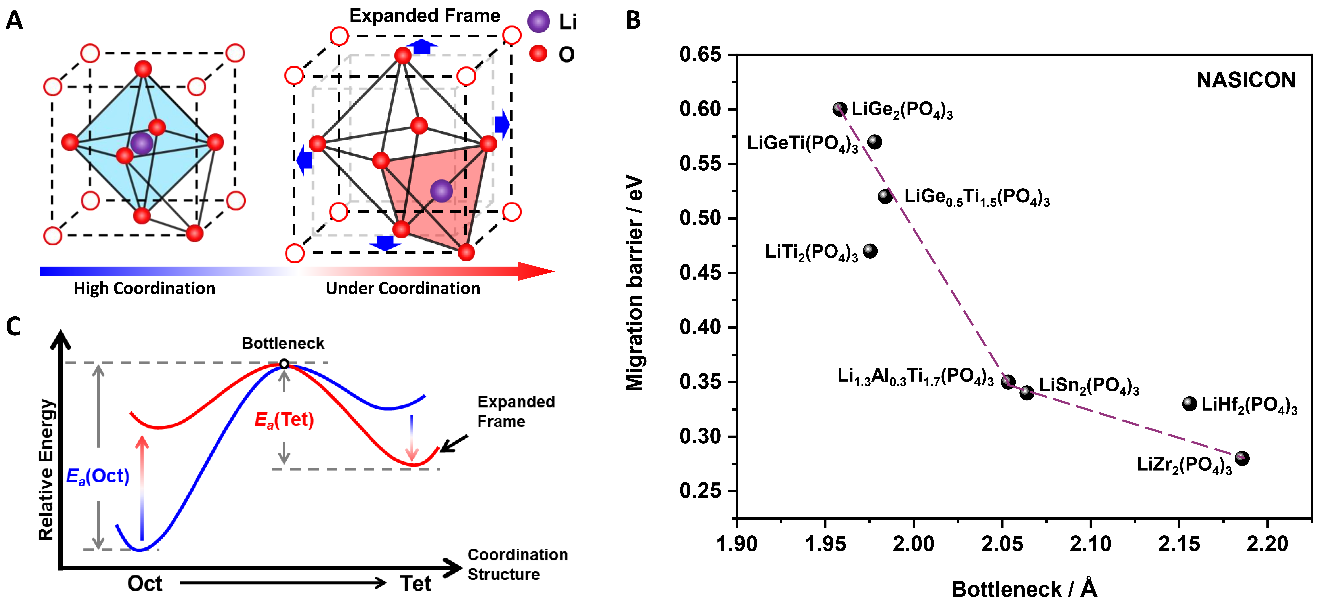
Fig. 1. Relationships among coordination modulation, bottleneck and migration energy barrier. (A) Structural schematic of coordination engineering for the transition between LiO6 hexahedrons and LiO4 tetrahedrons. (B) Migration barrier of Li+ ions from LiO6 hexahedrons to LiO4 adjacent tetrahedrons. (C) Relations between bottleneck and the migration energy barrier of lithium-ion in NASICON-type lithium solid electrolytes.

Fig. 2. Crystal structures of NZSP and LZSP. (A) Refined powder synchrotron XRD and (B) NPD patterns of NZSP and LZSP powders with Rietveld method. The SEM images of NZSP and LZSP are inserted in B. All these patterns are recorded at room temperature. (C) The retained polyhedral skeletons containing the evolution path of Na+ ions to Li+ ions. (D) The evolution of Na+ ions to Li+ ions polyhedrons within unchanged skeleton.
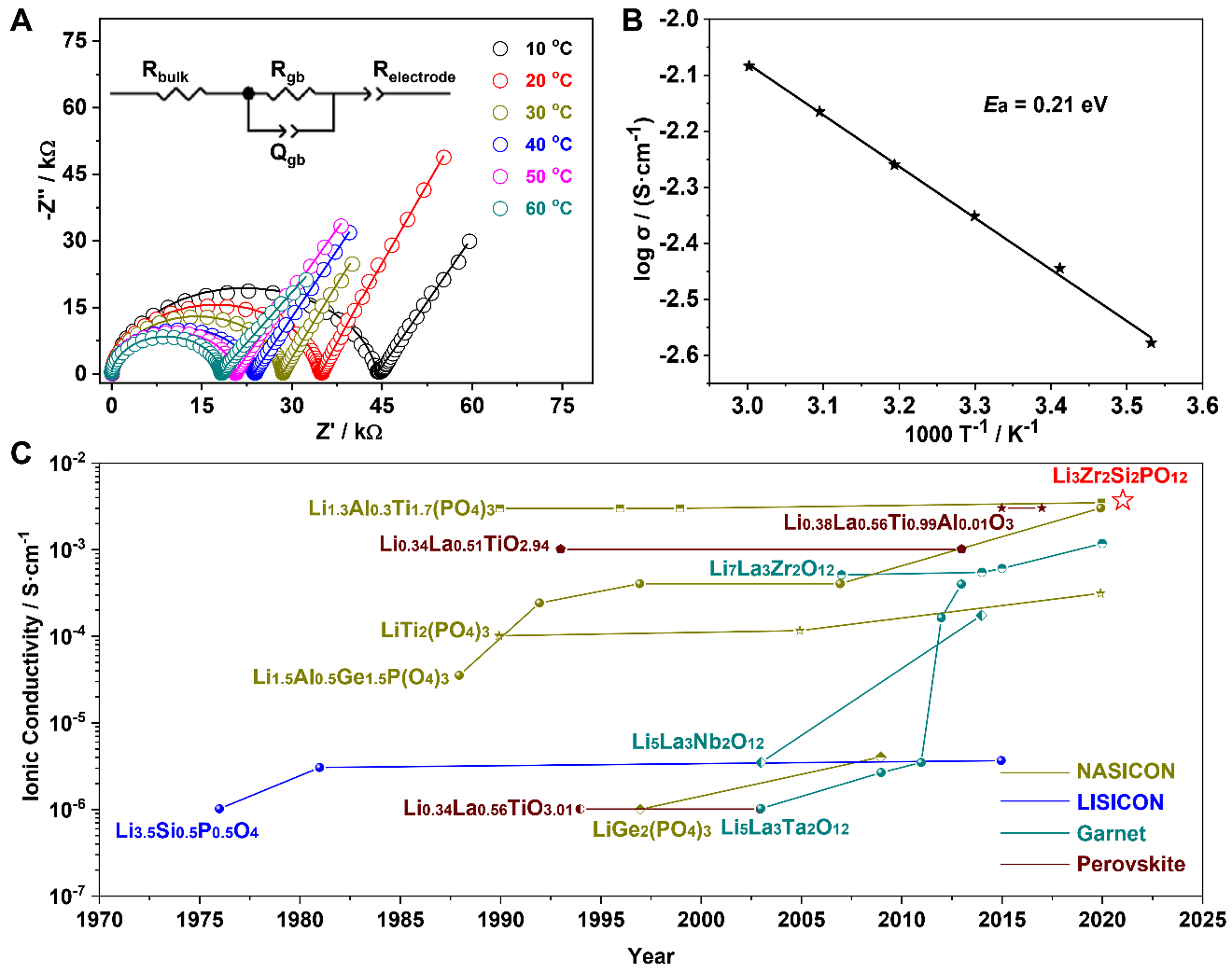
Fig. 3. Comparison of LZSP with other un-doped oxide solid electrolytes on ionic conductivities. (A) Nyquist plots of LZSP pellets from 10 to 60 °C; (B) Arrhenius plot of the ionic conductivity of LZSP; (C) Summary of bulk ionic conductivities of oxide solid electrolytes at room temperature, the materials are extracted from previous studies.

Fig. 4. Single-ion migration mechanisms of Na+-ion and Li+-ion from CI-NEB. (A) Energy barriers for single-ion migration of Na+-ion following the NaII-NaIV-NaI-NaIV-NaIII trajectory. (B) Energy barriers for single-ion migration of Li+-ion following the LiII-LiIV-LiI-LiIV-LiIII trajectory. The blue and purple balls stand for Na+ ions and Li+ ions, respectively. The olive, light green, pink and sky-blue polyhedrons identify the NaIIO8 dodecahedron, LiIIO5 pentahedron, NaIVO4/LiIVO4 tetrahedrons and NaIO6/LiIO6/ NaIIIO6/LiIIIO6 octahedrons.
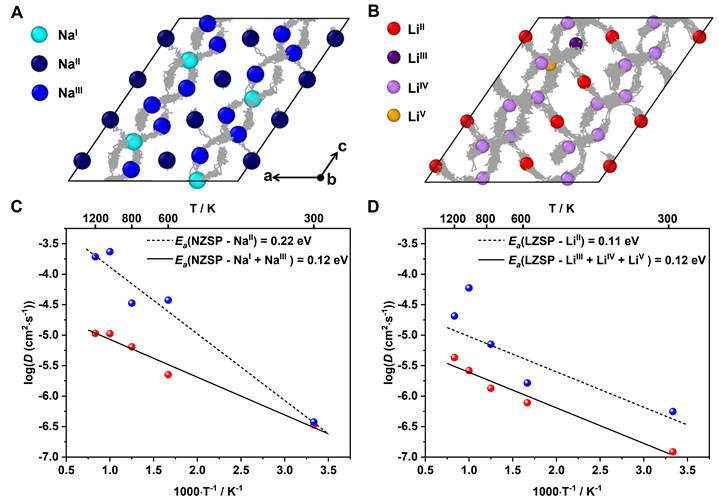
Fig. 5. Diffusion kinetics of NZSP and LZSP from AIMD simulations. (A) Diffusion pathways in monoclinic NZSP at 600 K. (B) Diffusion pathways in monoclinic LZSP at 600 K. (C) Arrhenius plot of Na+ diffusivity D as a function of temperature T in NZSP. (D) Arrhenius plot of Li+ diffusivity D as a function of temperature T in LZSP.
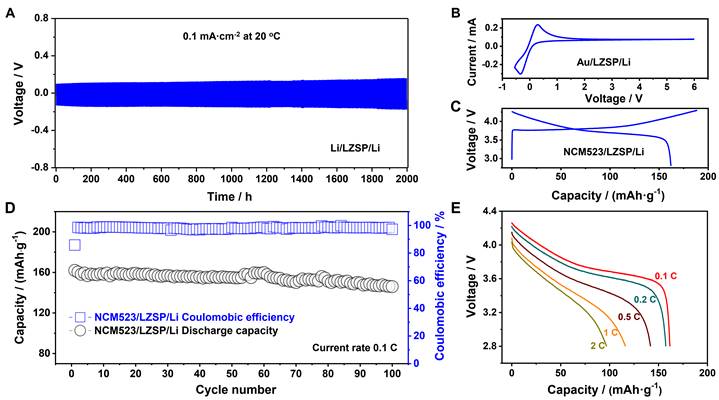
Fig. 6. The electrochemical performance of battery devices based on the LZSP solid electrolyte. (A) The cycling performance of a Li/LZSP/Li symmetric cell tested at a current density of 0.1 mA·cm-2. (B) The current-voltage curve of an Au/LZSP/Li cell. (C) The initial galvanostatic charge and discharge profile of a LiNi0.5Co0.2Mn0.3O2(NCM523)/LZSP/Li cell. (D) The cycling performance of a NCM523/LZSP/Li cell. (E) The rate performance of a NCM523/LZSP/Li cell.
Contact:
Prof. LIU Jianjun
Shanghai Institute of Ceramics
E-mail: jliu@mail.sic.ac.cn



